Abstract
Bosutinib (BOS) is a potent oral SRC/ABL tyrosine kinase inhibitor approved for treatment of adults with CML resistant or intolerant to prior therapy. Safety of BOS vs imatinib (IM) in 1st line treatment of patients (pts) with chronic phase (CP) CML was assessed in the ongoing, multinational, open-label, phase 3 BFORE study (NCT02130557). Here we further characterize GI and hematological safety profile of BOS.
BFORE randomized 536 pts 1:1 to 400 mg QD BOS or 400 mg QD IM. The safety population consisted of 268 (BOS) and 265 (IM) pts. Median duration of study treatment was 22.1 mo (BOS) and 21.7 mo (IM); 73% (BOS) and 68% (IM) of pts remained on treatment after a minimum of 18 mos follow-up. Treatment discontinuation rates included 15% (BOS) and 9% (IM) of pts discontinuing due to adverse events related to study drug and 4% (BOS) and 13% (IM) discontinuing due to suboptimal response/treatment failure.
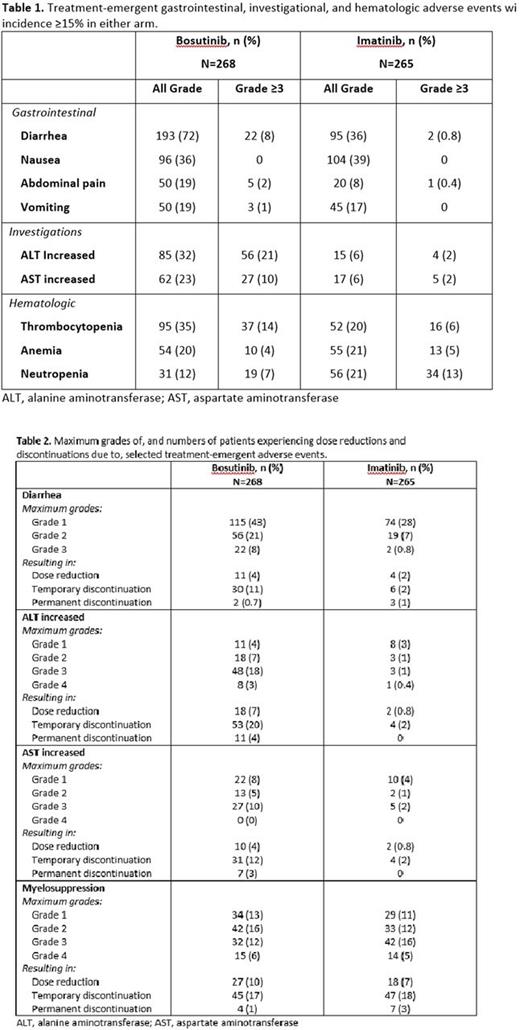
The most common treatment-emergent adverse events (TEAEs) were diarrhea (72%), nausea (36%), and thrombocytopenia (35%) for BOS and nausea (39%), diarrhea (36%), and muscle spasms (27%) for IM (Table 1). Diarrhea was mostly grade (Gr) 1 or 2 in both arms (Table 2). For BOS (n=193) and IM (n=95) pts with diarrhea, median times to first episode were 4 d (range, 1-721 d) and 32.5 d (range, 1-587 d), and median duration of any-grade diarrhea was 2 d (range, 1-692 d) and 2 d (range, 1-623 d), respectively. Median duration of Gr 3 diarrhea was 6 d (range, 1-21 d) for BOS and 10 d (range, 4-16 d) for IM. Diarrhea was managed with medication in 62% (BOS) and 42% (IM) of affected pts and resolved in 76% (BOS) and 63% (IM). Of 30 (BOS) and 6 (IM) pts temporarily stopping due to diarrhea (Table 2), 27 (90%) and 4 (67%) pts, respectively, were rechallenged without permanent discontinuation due to diarrhea (63% BOS and 100% IM pts experienced recurrence). Two BOS pts (0.7%) and 3 IM pts (1.1%) permanently discontinued treatment due to diarrhea. No deaths occurred from GI toxicity in either arm.
Increased ALT and AST TEAEs occurred in 85 (32%) and 62 (23%) of BOS pts, respectively, and 15 (6%) and 17 (6%) of IM pts, respectively (Table 2). Median times to first increased ALT were 32 d (range, 10-498 d) for BOS (n=85) and 57 d (range, 1-337 d) for IM (n=15). Median times to first increased AST were 49.5 d (range, 13-757 d) for BOS (n=62) and 139 d (range, 1-366 d) for IM (n=17). Of 53 pts who temporarily discontinued BOS due to increased ALT (Table 2), 48 were rechallenged with BOS, with 42 (88%) rechallenged successfully. Of 31 pts temporarily stopping BOS due to increased AST, 27 were rechallenged with BOS, with 22 (81%) rechallenged successfully. Median cumulative duration of AST elevations was similar in BOS (55 d; range, 5-288 d) and IM (62 d; range, 14-302 d) arms. Among pts with increased AST, events resolved in 82% (BOS) and 88% (IM) of affected pts. Median cumulative duration of ALT elevations was longer in the BOS (80.5 d; range, 4-385 d) vs IM (51 d; range, 15-332 d) arms. Among pts with increased ALT, events resolved in 85% (BOS) and 87% (IM). Two pts (0.7%) taking BOS and 1 pt (0.4%) taking IM experienced Gr 3 hyperbilirubinemia. Permanent discontinuation of BOS occurred in 11 pts (4.1%) and 7 pts (2.6%) with elevated ALT or AST, respectively; none with IM. No deaths due to liver function abnormalities occurred in either arm.
Incidence and maximum grades of myelosuppression TEAEs (including hematopoietic cytopenias and anemia) were similar between arms (Table 2). Median times to first myelosuppression were 27.5 d (range, 1-505 d) for BOS and 31 d (range, 1-603 d) for IM. Median cumulative durations of myelosuppression were 62 d (range, 4-700 d) for BOS and 62.5 d (range, 2-658 d) for IM. Events resolved in 74% (BOS) and 60% (IM) of pts with myelosuppression. Myelosuppression led to permanent treatment discontinuations in 4 (1%) BOS and 7 (3%) IM pts. No deaths occurred from myelosuppression in either arm.
In conclusion, safety profiles for BOS and IM in BFORE were distinct and consistent with known, respective safety profiles. BOS was well tolerated and associated primarily with manageable and generally mild GI toxicity and liver function test elevations, managed effectively by dose modifications, treatment interruptions, and concomitant medications. BOS patients with increased ALT and AST were successfully rechallenged in >80% of cases. No new safety events were reported for BOS when used in the 1st line setting with 400 mg QD.
Mauro: Bristol-Myers Squibb: Consultancy. Gambacorti-Passerini: Pfizer: Consultancy, Honoraria, Research Funding; BMS: Consultancy. Deininger: Novartis: Consultancy, Research Funding; Incyte: Consultancy; Pfizer: Consultancy; BMS: Consultancy, Research Funding; Ariad Pharmaceuticals, Bristol Myers Squibb, CTI BioPharma Corp, Gilead, Incyte, Novartis, Pfizer, Celgene, Blue Print, Galena: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Celgene: Research Funding; ARIAD: Consultancy. Chuah: Chiltern: Honoraria; BMS: Honoraria, Other: Travel; Novartis: Honoraria; Avillion: Honoraria. Kim: Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Il-Yang: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Milojkovic: Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Incyte: Honoraria, Speakers Bureau; ARIAD: Consultancy, Honoraria. le Coutre: Novartis: Honoraria, Research Funding; BMS: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; ARIAD: Honoraria. García Gutiérrez: Incyte: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Reilly: Avillion LLP: Employment. Jeynes-Ellis: Avillion LLP: Employment, Equity Ownership. Crescenzo: Pfizer: Employment, Equity Ownership. Uehara: Pfizer: Employment, Equity Ownership. Leip: Pfizer: Employment, Equity Ownership. DeAnnuntis: Pfizer: Employment, Equity Ownership. Bardy-Bouxin: Pfizer: Employment, Equity Ownership. Hochhaus: Pfizer: Research Funding; MSD: Research Funding; ARIAD: Research Funding; Incyte: Research Funding; Novartis: Research Funding; BMS: Research Funding. Brümmendorf: Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Cortes: Pfizer: Consultancy, Research Funding; Sun Pharma: Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal